Nutritional Quality
We eat food for pleasure and/or to obtain the important nutrients to be used as energy, building the body or stay healthy. Our research covers different aspects of nutritional quality: the micro- and macronutrients as well as antinutrients present in foods, the digestion in the gastrointestinal tract (GIT), and the gut microbiome. Nutritional quality depends on the type and amount of nutrients, but also how they are digested, released and absorbed during their journey through our mouth, stomach, small intestine, and finally ending in the colon.

Nutritional quality is an important factor in food choices, especially in the view of a growing request for convenient and healthy foods. However, the nutritional value of such foods is not only dependent on the amount of the nutrients, but also on the bioavailability of them. Before becoming bioavailable, compounds must be released from the food matrix and broken down in the GI tract to be ready for absorption by the body.
Many parameters determine the efficiency of the GIT to prepare for absorption. These include the type of raw material, the processing of raw material into food products, the structure of the food product, interaction of food components with other micro and macro-molecules, and the food breakdown in the mouth and in the stomach. Understanding the parameters responsible for digestibility is necessary to produce food products with high nutritional value.
The group has in-depth expertise in identifying processing-induced modifications of nutrients and changes in their structure and stability during digestion and absorption. In addition, the group has access to state-of-the-art analytical methods for assessing food quality parameters.
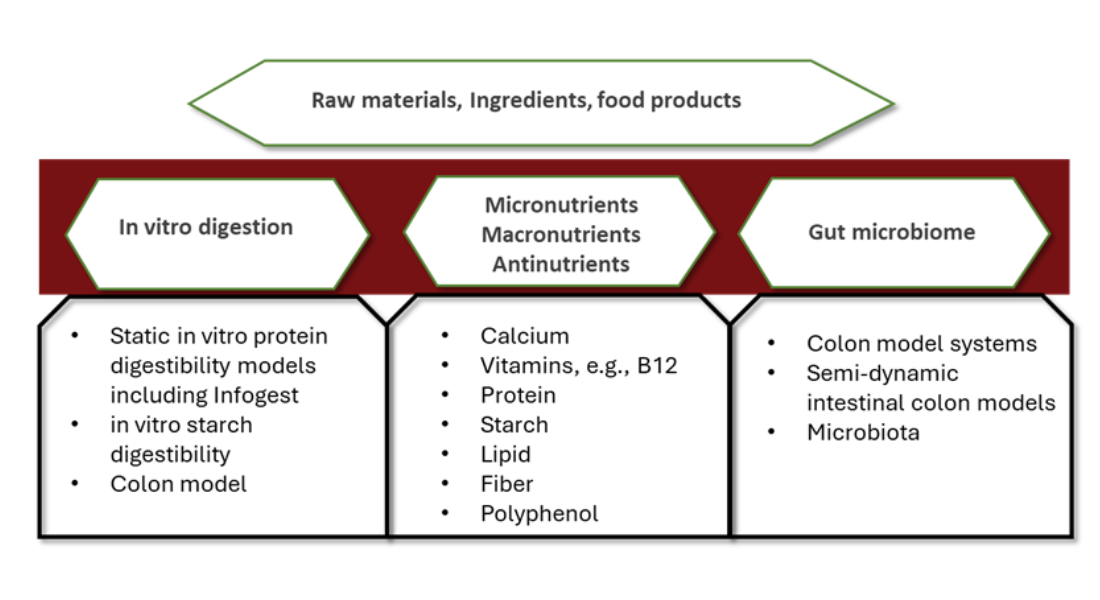
The link between food, nutrient uptake and health is the gastrointestinal tract (GIT). Digestion starts in the mouth, where the food is broken down, and continues through the GIT, where the nutrients are released and absorbed. However, not all types of nutrients are released and absorbed in the same manner. Therefore, in vitro digestion (IVD) is a useful tool to investigate and understand this link and a central factor in the development of healthy foods.
![]() Nutritional quality is an important factor in food choices, especially in view of a growing request for convenient and healthy foods. However, the nutritional value of such foods is not only dependent on the amount of the nutrients, but also on the bioavailability of them. Before becoming bioavailable, compounds must be released from the food matrix and broken down in the GI tract to be ready for absorption by the body. Many parameters determine the efficiency of the GIT to prepare for absorption. These include the type of raw material, the processing of raw material into food products, the structure of the food product and the food breakdown in the mouth and in the stomach. Understanding the parameters responsible for digestibility is necessary for the production of food products with high nutritional value.
Nutritional quality is an important factor in food choices, especially in view of a growing request for convenient and healthy foods. However, the nutritional value of such foods is not only dependent on the amount of the nutrients, but also on the bioavailability of them. Before becoming bioavailable, compounds must be released from the food matrix and broken down in the GI tract to be ready for absorption by the body. Many parameters determine the efficiency of the GIT to prepare for absorption. These include the type of raw material, the processing of raw material into food products, the structure of the food product and the food breakdown in the mouth and in the stomach. Understanding the parameters responsible for digestibility is necessary for the production of food products with high nutritional value.
The IVD Platform at FOOD covers a wide range of static and dynamic areas, see boxes below.
Diets inextricably link human health, ethics and environmental sustainability. Consumer’s concern to get enough nutrients has emerged entering the millennium along with the broad variety of various diets. The aim of food specification nowadays is to support the dietary recommendations at an individual level.
We provide knowledge about the relationship between protein source, processing and in vitro protein digestibility (IVPD).
Our IVPD research covers many areas to assess the nutritional benefits:
- INFOGEST method for validated IVPD measurement.
- Optimised and focused IVPD-model systems for e.g. screening of digestibility.
- Evaluation of IVPD of plant materials, like legumes, cereals and novel materials/sources, upon processing into food materials, e.g. protein extraction methods, like wet extraction and dry fractionation, to obtain protein-rich ingredients.
- Assessment of IVPD of plant-based products after processing, like extrusion, high pressure and fermentation, in order to clarify concepts and opportunities to increase the digestion potential of the products, e.g. meat analogues and meat substitutes.
- Assessment of IVPD of animal-based materials including side-streams upon processing into food ingredients and products.
- Assessment of IVPD of hybrid plant- and animal-based food products.
- Provide detailed insight and increased scientific knowledge about proteins and processing technologies in relation to consumption and nutritional quality and value.
The GI tract is the main site for food digestion and nutrients absorption. Protein quality is related to the capacity of the protein sources and diets to meet the specific nutritional requirements and satisfy the metabolic needs of a person for maintenance in a particular physiological state. Thus, digestibility of a protein plays an important role in determining its nutritional value, which constitutes an indicator of the efficiency of the digestive process related to either whole food or to a specific nutrient. Digestibility of a protein is closely related to the amino acid bioaccessibility. Before the amino acid becomes accessible they must be released from the food matrix in the GI tract.
Proteins are one of the important functional components for the body. Apart from their nutritional properties, proteins also possess functional properties that play an important role in food formulation and processing. The exact functional property of a specific type of protein are the individual physical and chemical properties utilised during processing. Different proteins may be added to foods to provide specific desired functional attributes and/or increase the nutritional value. Although proteins are found in most foods, their very dissimilar digestibility must be taken into consideration when addressing the nutritional value of a food product.
Plant materials, like cereals and legumes, may constitute a major source of proteins for human nutrition. However, plants contain the so-called anti-nutritional factors (ANFs). ANFs are phytochemicals or secondary metabolites synthesized in plants typically as self-defence against various inherently pathogens, insects and herbivorous. The major ANFs found in plant materials includes protease inhibitors, amylase inhibitors, polyphenols, phytic acid, glycosides, alkaloids, lectins, non-protein amino acids, oxalic acids, and triterpenes. In general, the ANFs reduce the nutritional value of the plant material by interfering with protein and starch digestibility as well as mineral absorption, or by forming toxic compounds in the body. Therefore, these ANFs can result in negative effects on gastric metabolism, so knowledge about using plant proteins in foods in relation to protein digestion is needed to support the green transition.
Food processing often result in protein changes necessary for utilising their functional capacity. However, food processing may also affect protein digestibility. On the one hand, protein denaturation can facilitate access of enzymes to the (targeted) peptide bonds in the protein, thus, increase digestibility. On the other hand, denaturation may also facilitate protein aggregation, thereby hindering proteolysis due to reduced access to the peptide bonds, hence reducing protein digestion. Moreover, other matrix effects, like protein interactions with starch and fibers, may affect enzyme accessibility and, thus, play a crucial role in protein digestibility. Combining different protein sources may improve (synergy) or hamper (antagonism) protein digestibility and the effect depends on several factors such as protein content, total amounts and relative mixing ratios, as well as other constituents.
Examples of papers:
V. Orlien, K. Aalaei, M.M. Poojary, D.S. Nielsen, L. Ahrné, J.R. Carrascal (2021). Effect of processing on in vitro digestibility (IVPD) of food proteins. Critical Reviews in Food Science and Nutrition, DOI:10.1080/10408398.2021.1980763
Joehnke MS, Jeske S, Ispiryan L, Zannini E, Arendt EK, Bez J, Sørensen JC, Petersen IL. 2021. Nutritional and anti-nutritional properties of lentil (Lens culinaris) protein isolates prepared by pilot-scale processing. Food Chemistry: X, 9, 100112, 9 pp
Martínez-Padilla E, Li K, Frandsen HB, Joehnke MS, Vargas-Bello-Perez E, Petersen IL. 2020. In Vitro Protein Digestibility and Fatty Acid Profile of Commercial Plant-based Milk Alternatives. Foods, 9, 1784, 17 pp
Vogelsang-O’Dwyer M, Petersen IL, Joehnke MS, Sørensen JC, Bez J, Detzel A, Busch M, Krueger M, O’Mahony JA, Arendt EK, Zannini E. 2020. Comparison of dry fractionation and isoelectric precipitation for production of faba bean protein ingredients: techno-functional, nutritional and environmental performance. Foods, 9, 322-346
The digestive process transforms proteins contained in food to physiologically active compounds. In vitro digestion systems are useful simulation models and tools for monitoring and understanding this complex transformation during digestion. To study the protein digestion, the condition of the in vitro digestion system needs to be chosen carefully. The specific enzymatic action on the proteins has a great influence on the in vitro protein digestibility (IVPD). In vitro models simulating the human gastro-intestinal (GI) tract can be divided into two groups according to sample movement, namely static and dynamic in vitro protein digestibility models. Generally, static models are based on a single reactor, and the pH is fixed to physiological relevant values (pH 1-3 for stomach, pH 6-7 for the small intestine and around 7 for colon). Static models are generally easy to up-scale and can be applied for screening large amounts of samples. Different protocols and analytical assays simulating these different parts of the upper GI tract exist and can be used in accordance with the needed knowledge.
Throughout in vitro digestion, proteins are gradually hydrolyzed into polypeptides, peptides, and free amino acids, depending on the phase and duration of digestion. The extent of protein hydrolysis can be evaluated at different levels of digestion by analyzing changes in the composition of the proteins under investigation or accumulation of peptides and amino acids in the digested samples. Several qualitative and quantitative analytical techniques are available to assess IVPD. Some methods focus on evaluating the digestibility pattern, while others provide direct estimation of the degree of hydrolysis (Orlien et al., 2021). The application of in vitro protein digestibility assays can provide valuable preliminary evidence for amino acid bioaccessibility (corresponding to a precursor for amino acid bioavailability) including specific interactions and associations between other nutrients in the food matrix as well as the underlying mechanisms. This knowledge can be used in an intelligent and targeted food formulation and processing regime.
Our IVPD models cover:
-
INFOGEST method for validated IVPD measurement.
-
Optimised and focused IVPD-model systems for digestibility screening of e.g. plant based ingredients and plant based foods.
The INFOGEST-protocol is a standardized protocol used in numerous laboratories. In short, in the oral phase simulated salivary fluid (pH 7) is mixed with the food for 2 min, this oral bolus is mixed with gastric fluid (pH 3) and pepsin in the gastric phase for 2 h at 37 °C followed by the final intestinal phase in which the resulting gastric chyme is mixed with intestinal fluid (pH 7) and a pancreatin enzyme mix together with bile salts for 2 h at 37 °C. The concentration of small peptides (less than 6 kDa) and free amino acids is measured spectroscopic by o-phthalaldehyde (OPA) based on L-glutamic acid and expressed as concentration of primary amines in the digesta (R-NH2, L-glutamic equivalents). The digestibility, % IVPD, of the food sample is calculated as the degree of hydrolysis of the sample in relation to the fully hydrolysed sample.
The UCPH/FOOD-IVPD-protocol comprises a static enzymatic protein hydrolysis simulating a gastro-pancreatic protein digestion. Pepsin hydrolysis (1h) is the initial step, followed by pancreatic hydrolysis (1h). The pancreatic hydrolysis can be extended to also cover medium (+3 h) and long (+24 h) term digestion. The evaluation of hydrolysis is conducted by TNBS-based detection of free α-amino groups (IVPD % ≈ degree of hydrolysis %). Results can be complemented with SDS-PAGE and LC-MS analysis for identification and quantification of the released peptides of the digestive process.
The method has been optimized for high protein samples (>30% protein), low protein samples (<30%), and infant formula samples (different pH during the hydrolytic stages of digestion, compared to the “adult” methods). The assay runs with a constant protein amount and fixed enzyme-to-substrate (E:S) ratio during hydrolysis. The UCPH/FOOD-IVPD-protocol has been applied to numerous plant-based protein ingredients and plant-based foods.
Examples of papers:
V. Orlien, K. Aalaei, M.M. Poojary, D.S. Nielsen, L. Ahrné, J.R. Carrascal (2021). Effect of processing on in vitro digestibility (IVPD) of food proteins. Critical Reviews in Food Science and Nutrition, DOI:10.1080/10408398.2021.1980763
Alonso-Miravalles L, Barone G, Waldron D, Bez J, Joehnke MS, Petersen IL, Zannini E, Arendt EK, O'Mahony JA. 2021. Formulation, pilot-scale preparation, physicochemical characterization and digestibility of a lentil protein-based model infant formula powder. Journal of The Science of Food and Agriculture, DOI 10.1002/jsfa.11199, 11 pp
Joehnke MS, Jeske S, Ispiryan L, Zannini E, Arendt EK, Bez J, Sørensen JC, Petersen IL. 2021. Nutritional and anti-nutritional properties of lentil (Lens culinaris) protein isolates prepared by pilot-scale processing. Food Chemistry: X, 9, 100112, 9 pp
Hoehnel A, Bez J, Petersen IL, Amarowicz R, Juskiewicz J, Zannini E, Arendt E. 2020. Combining high-protein ingredients from pseudocereals and legumes for the development of high-protein hybrid pasta: Enhanced nutritional profile. Journal of the Science of Food and Agriculture, DOI 10.1002/jsfa.10994, 11 pp
Martínez-Padilla E, Li K, Frandsen HB, Joehnke MS, Vargas-Bello-Perez E, Petersen IL. 2020. In Vitro Protein Digestibility and Fatty Acid Profile of Commercial Plant-based Milk Alternatives. Foods, 9, 1784, 17 pp
Hoehnel A, Bez J, Petersen IL, Amarowicz R, Juskiewicz J, Arendt EK, Zannini E. 2020. Enhancing the nutritional profile of regular wheat bread while maintaining technological quality and adequate sensory attributes. Food & Function, 11, 4732-4751
Vogelsang-O’Dwyer M, Petersen IL, Joehnke MS, Sørensen JC, Bez J, Detzel A, Busch M, Krueger M, O’Mahony JA, Arendt EK, Zannini E. 2020. Comparison of dry fractionation and isoelectric precipitation for production of faba bean protein ingredients: techno-functional, nutritional and environmental performance. Foods, 9, 322-346
Vogelsang-O'Dwyer M, Bez J, Petersen IL, Joehnke MS, Detzel A, Busch M, Krueger M, Ispiryan L, O’Mahony JA, Arendt EK, Zannini E. 2020. Techno-functional, nutritional, and environmental performance of protein isolates from blue lupin and white lupin. Foods, 9, 230-253
Joehnke, M. S., Lametsch, R., & Sørensen, J. C. (2019). Improved in vitro digestibility of rapeseed napin proteins in mixtures with bovine beta-lactoglobulin. Food Research International, 123, 346-354. https://doi.org/10.1016/j.foodres.2019.05.004.
Joehnke, M. S., Rehder, A., Sørensen, S., Bjergegaard, C., Sørensen, J. C., & Markedal, K. E. (2018). In Vitro Digestibility of Rapeseed and Bovine Whey Protein Mixtures. Journal of Agricultural and Food Chemistry, 66(3), 711–719. https://doi.org/10.1021/acs.jafc.7b04681
Joehnke, M. S., Sørensen, S., Bjergegaard, C., Markedal, K. E., & Sørensen, J. C. (2018). Effect of Dietary Fibre Fractions on in Vitro Digestibility of Rapeseed Napin Proteins. Polish Journal of Food and Nutrition Sciences, 68(4), 335–345. https://doi.org/10.2478/pjfns-2018-0005
Contact:

 Mahesha Poojary
Mahesha Poojary
Associate Professor - Promotion Programme
mahesha@food.ku.dk
Lilia Ahrné
Professor
lilia@food.ku.dk
