Bugs4urate: Prebiotics and microbial therapeutics for personalized treatment of hyperuricemia preventing gout
The project proposes an innovative approach to lower serum urate levels and prevent gout by combining personalised nutrition with novel urate-lowering live microbial therapeutics.
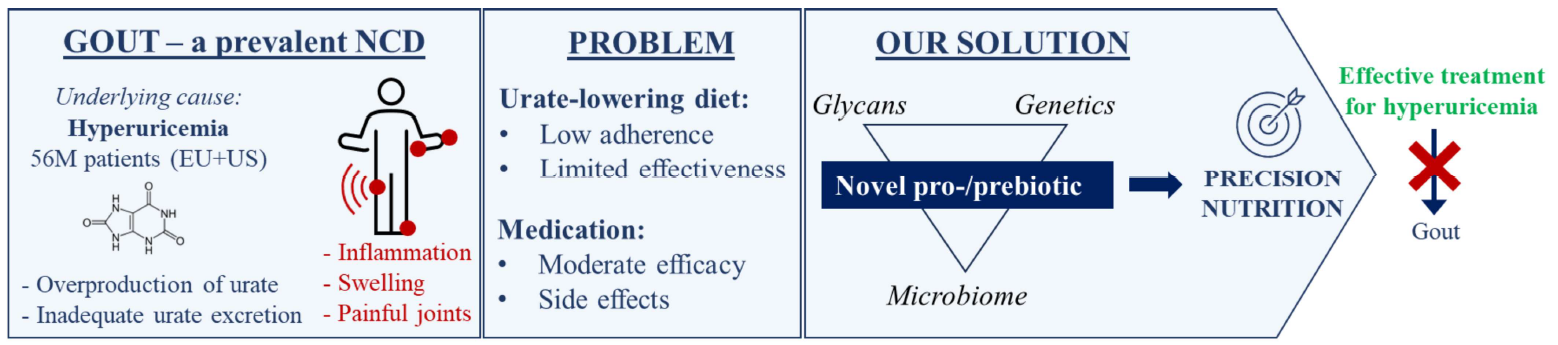
The Bugs4urate project is focused on understanding how diet, glycans, and the gut microbiome influence urate metabolism. By investigating these factors, we aim to provide scientific evidence for their role in treating hyperuricemia and preventing gout.
The Problem: Limited Treatment Options for Gout
Gout, a chronic noncommunicable disease (NCD), is caused by hyperuricemia — the accumulation of urate in the blood, due to an imbalance in the purine metabolism and/or inadequate renal excretion of the metabolic waste product urate. This imbalance in purine metabolism can result from diet or the body’s own metabolic processes and leads to urate crystallisation, causing painful gouty arthritis. Current treatments, such as strict dietary restrictions and medications like allopurinol and febuxostat, are often ineffective and may cause side effects. New strategies for prevention and targeted treatment of hyperuricemia are urgently needed.
The Bugs4urate Solution
The project proposes an innovative approach to lower serum urate levels and prevent gout by combining personalised nutrition with novel urate-lowering ingredients. This will address the limitations of current treatments through a precision nutrition tool using a combination of probiotics, dietary fibre and advanced responder stratification.
Three trials are planned at Örebro within this project. One ex vivo study to determine the most promising prebiotic to use in combination with the novel urate-lowering live microbial therapeutics. This will be followed by a clinical trial to assess the mechanisms involved in vivo after intake of the combined pre- and probiotic product. Finally, a larger 3-armed randomised, placebo-controlled clinical trial will assess the effect of the combined pre- and probiotic product in a hyperuricaemic adult cohort, to determine its potential in ameliorating the disease progression through urate-lowering properties. Multi-omics data will be collected throughout, in order to analyse personalised effects by the clinical product.
Chronic inflammation is a sum of a range of processes perturbing to the activation and inactivation of the immune system. One important factor in these processes is glycosylation. Glycans, or complex carbohydrates, decorate and modulate the majority of the cell surface and secreted proteins and form an essential basis for host-microbiome interactions in the gut. Here we will study the mucus glycome in the gut, associated with the microbiome composition, as well as the clinical trial participant’s blood glycoproteome as a measure for immune activation and responsiveness to microbial therapeutics.
Analytics for characterising the glycome is based on mass spectrometry. In this project we will both use state-of-the-art, as well as to develop new methods especially targeting the characterization of intestinal tissue and blood glycoproteome.
One size rearely fits all. This is true for life in general, and especially for complex traits such as hyperuricemia and gout, where lifestyle, diet, human genetics, and the gut microbiome as a totality are key in understanding both who will end up getting the diseases as well as who will likely benefit from a treatment targeting the gut. By collecting a wide array of clinical and omics layer data, researchers at FOOD (University of Copenhagen) will use machine learning and bioinformatics to integrate these data to further our general understanding of this disease as well as to provide means for responder stratification into those who likely will benefit from treatment as well as those who will not.
The live therapeutics has its target the small intestine, and the passage from mouth over the stomach is a hard journey. Hence optimizing the formulation of the therapeutic product by encapsulation with beta-glucans is key to ensure stability and efficiency of the product given to patients.
By optimising the culture conditions for the clinical product strains, combined with production and lyophilisation tests and screening for beta-glucans of interest, NeoBioSys will define the optimum conditions for producing the clinical product. The beneficial enzymatic activity of each strain administered to patients will be measured, as will the long-term stability of the clinical product.
Manipulating the interaction between the human body, the microbiome, and diet is a complex system that is hard to study in detail in vivo. We will use and develop ex vivo technology combining human cells and bio-fluids to understand how bacteria and diet perturbations change the conditions in the gut and its consequences on the human host to be able to provide mechanistic insights. Thus, a miniaturised gastrointestinal tract (GIT) platform will be developed integrating: 1) a digestion-chip, representative of the upper digestion, 2) a fermentation-chip to mimic the behavior and metabolic activity of microbiota representative of responder and non-responder signatures and test the effect of formulations; 3) a gut-chip to recreate the human intestinal epithelium including organoid-derived monolayers cells obtained from a specific patient. Validation will be based on testing the best-performing probiotic/prebiotic food supplement tested in clinical trials and comparing all the omics obtained from ex vivo and in vivo studies, before and after treatment.
The urate lowering bacterial therapy will be commercialised as a food-for-special-medical purpose (FSMP) in popular terms called medical foods.
The product commercialisation will require interactions with venture capitalists that support company scaling as well as food-ingredient producers and pharmaceutical companies with market access.
Researchers from Department of Food Science
Morten Arendt Rasmussen, mortenr@food.ku.dk
Ramus Riemer Jakoben, rasmus@food.ku.dk
External researchers
Robert Ian Brummer
Julia
Linnea
Rikke C Nielsen, info@beotherapeutics.com
Noortje de Haan, n.de_haan@lumc.nl
Catarina Gonçalves, catarina.goncalves@inl.int
Lorenzo Pastrana, lorenzo.pastrana@inl.int

Contact
Morten Arendt Rasmussen,
Professor,
mortenr@food.ku.dk

Bugs4Urate is a HORIZON Europe EIC Pathfinder challenges 2023 project.
The consortium consists of:
- University of Copenhagen
- Örebro University
- Leiden University Medical Center
- Neobiosys
- BEO Therapeutics
Funding
Period: 2024 - 2027
Amount: EUR 3,8 mil.
Source: European Innovation Council, Pathfinder Grant

